Intra-articular administration of a 2.5% polyacrylamide hydrogel (PAAG- Arthramid Vet) is shown to reduce or abolish lameness in distal interphalangeal (coffin) and metacarpo/ metatarso- phalangeal (fetlock) joints in horses undertaking equestrian disciplines. To date there had been no studies evaluating its’ efficacy in inter-carpal (knee) joints of racehorses conducted in a blinded fashion or up against standard treatments (controls).
Objectives:
To investigate the clinical efficacy of a 2.5% PAAG (Arthramid Vet) in the management of inter-carpal joint lameness in racing Thoroughbreds.
Study design:
Prospective double-blinded positive-control study. Read full article here.
Methods:
33 flat-racing Thoroughbreds in full training at a single training facility with lameness (AAEP 1-3/5) localised to the inter-carpal joint by intra-articular analgesia and radiological assessment were enrolled. Horses were randomly allocated to be treated intra-articularly with either 2ml of a 2.5% polyacrylamide hydrogel, 12mg of triamcinolone acetonide (cortisone) or 20mg of sodium hyaluron (Hyonate®- followed by 2 further intravenous treatments of 40mg, at weekly intervals), by the treating veterinarian. All horses were rested for 48 hours’ post-treatment and then re-entered an unaltered training regime. i.e. kept in full work.
Subsequent examinations at 2, 4, and 6 weeks were performed by the blinded examining veterinarian for all groups. Horses treated with the 2.5% PAAG (Arthramid Vet) were monitored through to 12 weeks for reoccurrence of lameness in the treated joint.
Results:
Compared to horses that received triamcinolone acetonide or sodium hyaluron, horses treated with 2.5% PAAG (Arthramid Vet) showed a greater chance of resolution of lameness, joint effusion and reaction to passive flexion at four (p<0.05) and six (p<0.05) weeks, with no difference seen between groups at 2 weeks. There was no significant difference between the triamcinolone acetonide and sodium hyaluronate groups at any time point. Two-thirds (67%) of horses treated with the 2.5% PAAG hydrogel were still lame-free at 12 weeks.
Conclusions:
This study indicates that a 2.5% PAAG (Arthramid Vet) hydrogel can be used in the management of inter-carpal joint lameness in flat-racing Thoroughbreds and was superior to and longer lasting than both Triamcinalone and Hyaluronic Acid. A reduction in joint effusion and reaction to passive flexion aligns with the proposed mode of action of Arthramid Vet.
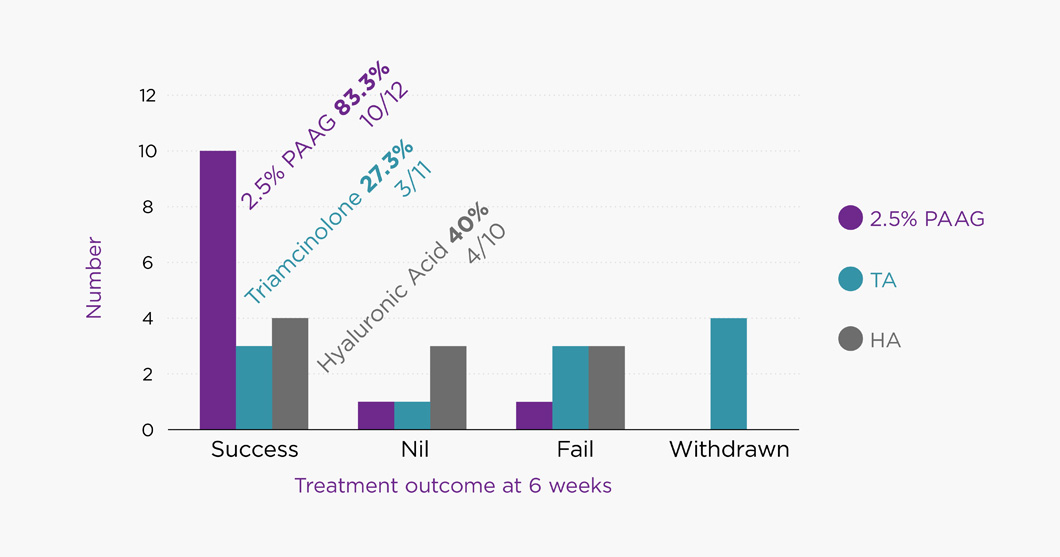
Table 1: Pre-admission variables per treatment group. There was no statistically significant different between treatment groups.
| PAAG | TA | HA | |
| Number | 12 | 11 | 10 |
| Age: range (mean) | 2-5 (3.08) | 2-4 (3) | 2-6 (2.8) |
| Sprinter (%) | 5 (42) | 5 (45) | 4 (40) |
| Miler (%) | 5 (42) | 4 (36) | 4 (40) |
| Stayer (%) | 2 (16) | 2 (19) | 2 (20) |
| Lameness range (mean) | 1-3 (1.67) | 1-4 (2.18) | 1-3 (1.8) |
| Effusion range (mean) | 0-2 (0.5) | 0-1 (0.1) | 0-2 (0.2) |
| Reaction to flexion range (mean) | 0-2 (0.67) | 0-2 (0.54) | 0-2(0.6) |
| Radiological score range (mean) | 0-2 (0.5) | 0-2 (0.63) | 0-2 (0.6) |