Use Of a 2.5% Cross-Linked Polyacrylamide Hydrogel In The Management Of Joint Lameness In a Population Of Flat Racing Thoroughbreds: A Pilot Sudy
Published in JEVS; Read here
Abstract
Osteoarthritis is one of the most common disease processes effecting equine athletes, causing up to 60 % of all lameness. This prospective longitudinal study reported on the effect of treatment of carpal and metacarpophalangeal (fetlock) joint lameness with 2.5% cross-linked polyacrylamide hydrogel (PAAG)- Arthramid Vet. A total of 49 flat-racing Thoroughbreds at a single training facility were included in the study. The results showed a significant improvement in lameness grades at weeks 1 (p<0.01), 4 (p<0.001), 12 (p<0.001), and 24 (p<0.001) when compared to baseline lameness at week 0. This pilot study suggested that 2.5% cross-linked PAAG is a safe and effective joint treatment for managing joint lameness in Thoroughbred racehorses.
Materials and Methods- A total of 49 horses were included in the study and selected from those presenting for routine veterinary care over a 12 month period at a single TB training facility. Horses had confirmed lameness isolated to the joint by clinical examination and intra-articular analgesia (nerve-blocks). Radiographs were taken and any horses excluded with fractures or osteochondral fragments. Other exclusion criteria included concurrent joint treatments or having undergone surgery within 3 months of the study.
Horses were treated with 2mls Arthramid Vet (2.5% PAAG) on Day 0 and followed up at weeks 1, 4, 12 and 24. All horses were rested for 24 hours immediately after treatment before re-entering an unaltered training regimen.
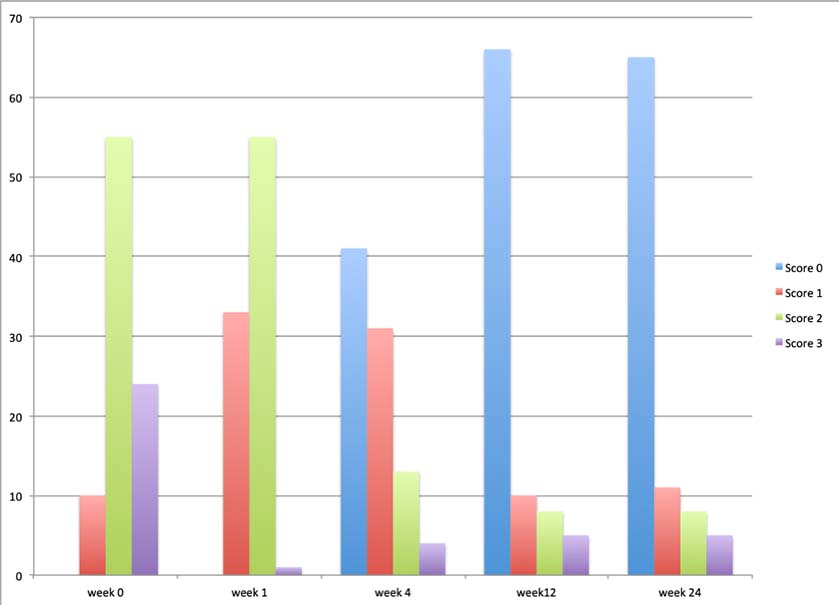
Results- The percentage of lame-free horses at each evaluation point was 0% at 1 week, 43% (21/49) at 4 weeks, and 67.3% (33/49) at 12 weeks. One horse regressed after that time so at 24 weeks 65.3% (32/49) of the horses were lame free, and a further 14.3% (7/49) had improved to some degree and enough to remain in race training. The distribution of the change in lameness grades for individual joints over the observed time periods is shown in Figure 1. The largest reduction in lameness scores occurred at 4 weeks, with some taking up to 12 weeks after treatment to respond, and no further improvement in lameness between 12 and 24 weeks was observed. No side effects or adverse reactions were observed in any of the treated joints.
Results of the final statistical model showed a statistically significant improvement in lameness grades at weeks 1 (p<0.01), 4 (p<0.001), 12 (p<0.001), and 24 (p<0.001) when compared to week 0. A positive response to flexion tests was also associated with higher lameness grade (p < 0.01) in the final model.The random effect for horse was significant in the final regression model.
Table 1. Summary of Horses and Results
| Variable | Number (%) |
| Thoroughbred race horses included | 49 |
| Total number of joints injected | 89 |
| Age range (years) | 3-7 |
| Mean age (years) | 5 |
| SD (years) | 1.68 |
| Limb Involved | |
| Fore | 89 (100%) |
| Hind | 0 |
| Joint involved | |
| Metacarpophalangeal | 10 (11%) |
| Intercarpal | 78 (88%) |
| Radiocarpal | 1 (1%) |
| Lameness scoring at baseline | |
| 0 | 0 (0%) |
| 1 | 3 (6%) |
| 2 | 22 (45%) |
| 3 | 24 (49%) |
| 4 | 0 (0%) |
| 5 | 0 (0%) |
| Reaction to flexion test score at 24 weeks | |
| 0 (none) | 76/89 (85%) |
| 1 (mild) | 13/89 (15%) |
| 2 (moderate) | 0 (0%) |
| 3 (severe) | 0 (0%) |
| Horses free of lameness (grade 0) | |
| 0 week | 0 (0%) |
| 1 week | 0 (0%) |
| 4 weeks | 21 (42.9%) |
| 12 weeks | 33 (67.3%) |
| 24 weeks | 32 (65.3%) |
Figure 1. Significant improvement.
Graph showing significant improvement (p<0.0001) in lameness scores following treatment with 2.5% PAAG between 4 and 24 weeks compared to baseline lameness at Day 0. y-axis = % of horses in each lameness grade (score 1,2 or 3 out of 5).
Conclusion
This study shows Arthramid Vet is a safe and practical first line treatment option for joint lameness isolated to the metacarpophalangeal (fetlock) and carpal joints of TB race horses. The percentage of horses free of lameness at 4 weeks and 24 weeks post-injection was 43% and 62% respectively, following a single intra-articular injection of 2 mL of a 2.5% cross-linked PAAG.
Findings indicated that it took between 1 to 12 weeks to respond to treatment, with the greatest response occurring by 4 weeks. This indicates that re-examination at 4 to 6 weeks in an important time point to evaluate response to treatment as it is possible that the dosage used was insufficient to restore pain free motion.