New hydrogel technology, researched and adapted for veterinary use, offers a pioneering treatment for osteoarthritis in horses without an active pharmaceutical.
Osteoarthritis (OA) is often cited as the most significant musculoskeletal disorder in both humans and horses. Clinically, it is associated with pain, lameness and dysfunction of the affected joint and around 60% of all equine lameness is due to OA (1).
The rapid resolution of lameness by a reduction in pain is paramount, but treatment should ideally also serve to arrest or slow the progression of disease (2). A new non-pharmaceutical technology provides veterinarians a pioneering leap forward in the treatment of this debilitating disease in animals. This novel product is Arthramid® Vet, a 2.5% (25mg/mL) IL-X Cross-linked Polyacrylamide (PAAG) Hydrogel now registered in Australia and New Zealand for the intra-articular treatment of non-infectious causes of lameness in horses. Arthramid® Vet is a Schedule 4 Prescription Animal Remedy yet contains no pharmaceutical active.
In its natural state, Arthramid® Vet is an inert, non-pyrogenic and neuro- innocuous porous polymer hydrogel consisting of 97.5% sterile water and 2.5% cross-linked polyacrylamide.
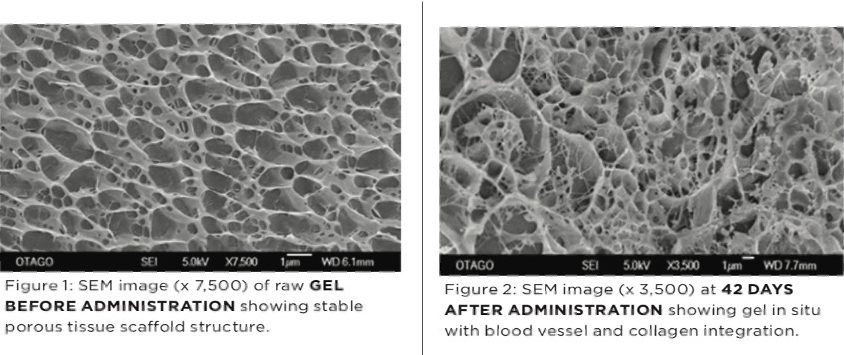
Following intra-articular injection Arthramid® Vet adsorbs into the synovial membrane over a period of 2 to 4 weeks, to become fully incorporated into the sub-intimal layer of the synovium through a process of cell migration and vessel ingrowth [see Figure 2 below]. It is safe, biocompatible and non-degradable, creating permanent bonds with its host tissue and providing powerful mechanical and disease-modifying properties (3).
Arthramid® Vet acts as a dynamic tissue scaffold forming a thick, cushion-like membrane that increases the elasticity and tensile strength of the joint capsule. A new and hypercellular synovial cell lining also forms facing the joint cavity. This precise therapeutic action on the synovium improves its capacity to transfer load, modifies the disease process and improves joint function (3,4).
Multiple studies worldwide have shown that Arthramid® Vet resolves OA lameness in 66 – 83% of horses (5-9). A 2018 Australian study in racing Thoroughbreds showed that Arthramid® Vet resulted in 83.3% of horses being sound at 6 weeks in the face of ongoing exercise (pending publication). That is, the total elimination of lameness in 83.3% of horses, not just a degree of improvement. In contrast, the study’s alternative treatment groups showed at 6 weeks only 40% were sound after hyaluronic acid (HA) and 27.3% after triamcinolone (TA) – see Graph at top of page. 2.5% PAAG has also been used to manage osteoarthritis in dogs and cats and has recently been approved for use in human knee osteoarthritis in Europe. Whilst formal research on the use in companion animals is limited, positive results have been reported in the treatment of OA in elbows, hips and stifles and data collection continues with results pending publication.
Arthramid® Vet is a ground-breaking new technology that allows progressive veterinarians the ability to manage lameness more effectively for longer and without any active pharmaceutical. Whether the greater reward is less lameness, improved animal welfare or reduced drug administration, this long-term non-pharmaceutical OA treatment ticks many boxes.
This sponsored article recently appeared in the Australian Vet Practice Magazine. https://vetpracticemag.com.au/arthramid-vet/
References
- McIlwraith, C.W., Principles and practices of joint disease treatment. In: Ross, M.W., Dyson, S.J., editors. Diagnosis and management of lameness in the horse. 2nd edition. Saunders. Missouri, 2011; 840-852.
- Caron, J.P., Intra-articular injections for joint diseases in horses. Vet Clin North Am Equine Pract. 2005; 21: 559-573.
- Christensen, L.H., Camitz, L., Illigen, K.E., Hansen, M., Sarvaa, R., and Conaghan, P.G. Synovial incorporation of polyacrylamide hydrogel after injection into normal and osteoarthritic animal joints. Osteoarthritis and Cartilage 2016; 24: 1999-2002.
- Tnibar, A., Persson, A.B., and Jensen, H.E. Mechanisms of Action of an Intraarticular 2.5% Polyacrylamide Hydrogel in a Goat Model of Osteoarthritis: Preliminary Observations. SM J Biomed Eng 2017; 3(3): 1022.
- Janssen I, Koene M and Lischer C. Intra-articular application of polyacrylamide hydrogel as a treatment of osteoarthritis in the distal interphalangeal joint: case series with 12 horses. Pferdeilkunde 2012; 28(6): 650-656.
- Tnibar A, Schou- gaard H, Koene M and Markussen B. A controlled clinical trial on the efficacy of an intra-articular polyacrylamide hydrogel in horses with osteoarthritis. Proceedings of the European College of Veterinary Surgeons Annual Scientific Meeting 2014; 3-5th July, Copenhagen, Denmark.
- Tnibar A, Schougaard H, Camitz L, Rasmussen J, Koene M, Jahn W and Markussen B. An international multi-centre prospec- tive study in the efficacy of an intraarticular polyacrylamide hydrogel in horses with osteoarthritis: a 24 months follow-up. Acta Veterinaria Scandinavia 2015; 57:20.
- Bathe, AP, Read RM and Briggs C Intra-articular polyacrylamide hydrogel for the treatment of 20 horses with non-responsive osteoarthritis of the interphalangeal joints: a prospective study. Veterinary Orthopaedic Society 43rd Annual Conference Abstract 2016; USA.
- de Clifford LT, Lowe JN, McKellar CD, Bolwell C and David F. Use of a 2.5% cross-linked polyacrylamide hydrogel in the management of joint lameness in a population of flat racing Thoroughbreds: A pilot study. Journal of Equine Veterinary Science 2019; 77: 57-62.